In December 2014, the Food and Drug Administration issued a Class I recall for the Alere INRatio and INRatio2 monitor system and test strips. Alere admitted to receiving almost 19,000 complaints of malfunction with its INRatio monitor system and test strips, which are linked to six serious injuries and three deaths. Users report they received erroneously low PT/INR readings from the Alere INRatio, putting them at risk for serious injury or death.
For patients taking the blood thinning drug Warfarin, monitoring their PT/INR levels is an important part of their therapy to prevent both stroke or a serious bleeding event. Warfarin helps prevent dangerous blood clots by thinning the users blood. Additionally, these patients need to keep their clotting time, or PT/INR, at a therapeutic level to prevent a serious bleeding injury.
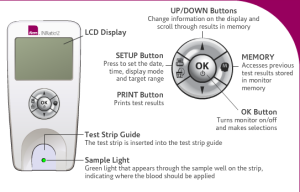 The Alere INRatio PT/INR monitor system and test strips were developed and marketed as a convenient, home-based method to report PT/INR levels to physicians. Physicians adjust a patient’s warfarin regimen to maintain a proper PT/INR level with the information provided by the Alere INRatio. Alere’s website for its INRatio2 home monitor system advertises the alleged simplicity and convenience of patients performing PT/INR self-testing.
The Alere INRatio PT/INR monitor system and test strips were developed and marketed as a convenient, home-based method to report PT/INR levels to physicians. Physicians adjust a patient’s warfarin regimen to maintain a proper PT/INR level with the information provided by the Alere INRatio. Alere’s website for its INRatio2 home monitor system advertises the alleged simplicity and convenience of patients performing PT/INR self-testing.
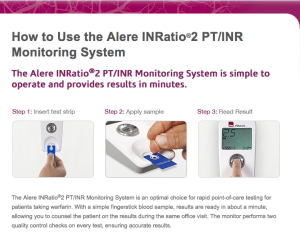
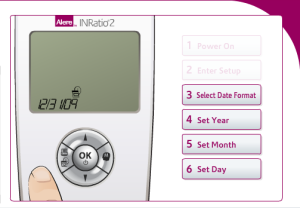
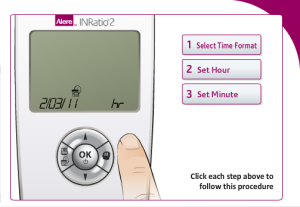
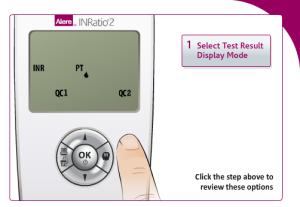
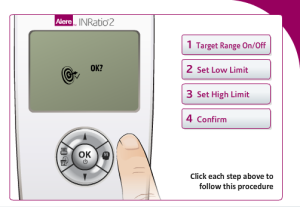
Performing the initial setup for the Alere INRatio2 Monitoring System
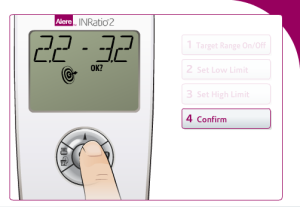
Alere claims performing self-testing to be a simple three step process on its website for users. The INRatio2 monitor system requires patients to format their INRatio PT/INR monitor in order to perform self-testing. These steps include:
- Formatting the Date:
- Formating the Time:
- Display mode of test results:
Additionally, Alere INRatio2 users must choose whether to select a target range for their INR readings and if so they must enter the low and high limits for the target parameters.
Is performing PT/INR self-testing with the INRatio2 really as easy as 1-2-3?
 Once the setup is complete, users are ready to utilize their Alere INRatio to perform self-testing. While Alere advertises the INRatio as a simple three step process, there are several other intermediary actions which must be taken to receive a PT/INR reading.
Once the setup is complete, users are ready to utilize their Alere INRatio to perform self-testing. While Alere advertises the INRatio as a simple three step process, there are several other intermediary actions which must be taken to receive a PT/INR reading.
Users must perform the following (note: this is NOT medical advice and we strongly recommend speaking with your doctor if you use the Alere INRatio or INRatio2 PT/INR Monitoring System):
INRatio Step 1
Turn on the monitor:
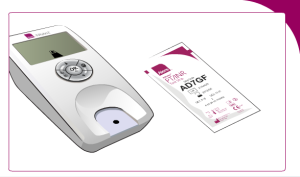
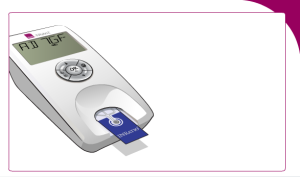
Insert a test strip:
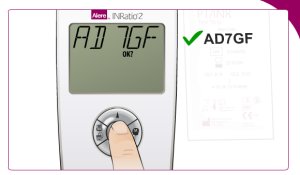
Enter the code found on the test strip package:
INRatio Step 2
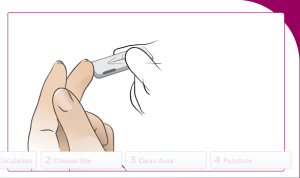
Prepare the finger stick to draw blood:
Increase blood circulation to the fingers:
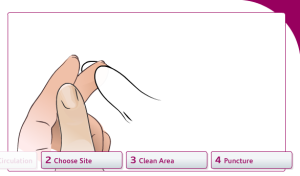
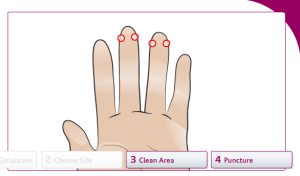
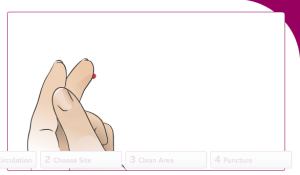
Choose a site to draw blood from:
Clean the selected area with 70% isopropyl alcohol:
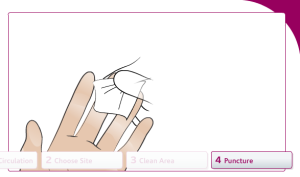
Puncture the finger and draw blood:
Directly apply the blood sample to the INRatio2:
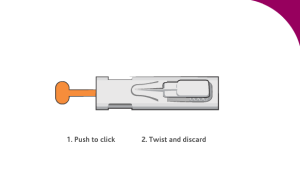
Dispose of the materials:
INRatio Step 3
If the blood sample applied is accepted by the Alere INRatio2, the device will emit a beeping sound and provide a reading in under two minutes. If no errors are reported by the INRatio2, the user may report this reading to their physician. The reading will be displayed based on the formating performed by the user.
Users are also advised to perform routine cleaning of their INRatio2 monitor with a damp cloth or cotton swab with 70% isopropyl alcohol or another mild detergent:
What kind of errors with the INRatio does Alere alert users to be aware of?
The Alere INRatio monitor system does not always work as intended and can be prone to user error. Several factors may contribute to users of the Alere INRatio receiving an error message, including failure to follow these steps:
- The monitor and test strips should be at room temperature before use
- Room temperature should be between 50 degrees Fahrenheit and 95 degrees Fahrenheit
- Relative humidity should be between 10% and 95% without condensation for testing
- The monitor should be transported in its original shipping container or another secure container. Avoid dropping the Alere INRatio2 monitor or treating it roughly
- Test with the monitor on a level surface
- Do not touch or move the INRatio2 monitor during testing
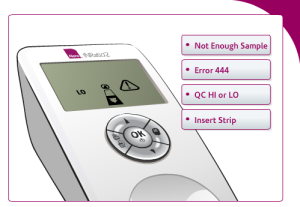
The types of error messages users can receive as a result of not following directions include:
Not enough sample: This message may appear when not enough blood reaches the test area. This can occur if not enough blood was applied to the sample well or if the strip channel is blocked.
 Error 444: Users may receive this error if there is a problem with the monitor calculating PT/INR levels. It may occur if not enough blood is applied to the test strip or there is a problem with the monitor or test strip itself.
Error 444: Users may receive this error if there is a problem with the monitor calculating PT/INR levels. It may occur if not enough blood is applied to the test strip or there is a problem with the monitor or test strip itself.
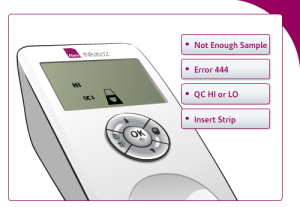 Error QC HI or LO: This error message may appear if the onboard controls are out of range. This may happen if the test strip was exposed to humidity, is expired, or the user applied poor technique in performing the self-test.
Error QC HI or LO: This error message may appear if the onboard controls are out of range. This may happen if the test strip was exposed to humidity, is expired, or the user applied poor technique in performing the self-test.
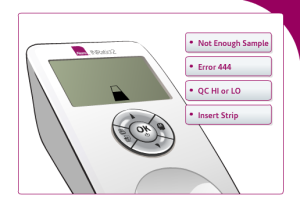 Insert Strip: The INRatio2 monitor may display this image if users have failed to properly insert the test strip. Users of the INRatio2 may accidently insert the test strip upside down or not far enough into the monitor.
Insert Strip: The INRatio2 monitor may display this image if users have failed to properly insert the test strip. Users of the INRatio2 may accidently insert the test strip upside down or not far enough into the monitor.
Alere INRatio users have reported receiving false PT/INR readings even when an error message is not displayed. In response, the FDA recalled all monitor systems and test strips manufactured between April 2008 and December 2014. The Class I recall issued for the Alere INRatio monitor system and test strips is the most serious the Food and Drug Administration may issue. Medical devices under Class I recall are deemed likely to contribute to serious injury or death if used.
What can I do if I’ve been seriously injured by an Alere INRatio monitor system?
If you or a loved one suffered a serious bleeding injury or death after receiving an erroneously low PT/INR reading from an Alere INRatio or INRatio2 PT/INR monitor system, you may be entitled to substantial compensation. The Cochran Firm, D.C. has a team of experienced and dedicated attorneys actively investigating injury claims related to the defective Alere INRatio monitor system and test strips.
We can help you recover hospital bills, lost wages, pain and suffering, and other damages. There are strict deadlines when filing an injury claim so we ask that you please contact us at your earliest convenience. Call us at 202-682-5800 locally or at 1-800-THE-FIRM (843-3476) to reach us 24/7. You may also fill out a contact form on our website to receive a free, prompt, and confidential case review.
We are offering free consultations to all individuals (and their family members) who have suffered bleeding injuries after receiving false INR reading from the Alere INRatio or INRatio2 PT/INR monitor. We represent individuals on a contingency basis, meaning there are no fees unless we recover for you. You pay nothing if we do not win your case.