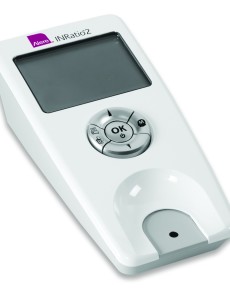
Cochran Firm lawyer discusses Alere INRatio lawsuit and recall in TV interview
David Haynes, The Cochran Firm, D.C.’s managing attorney, was recently interviewed on Ring of Fire about Alere INRatio lawsuits and the FDA recall of the home PT/INR monitoring device. Ring of Fire is broadcast throughout the United States on Free Speech TV and also distributed on radio stations throughout the country. Mike Papantonio is a senior partner…