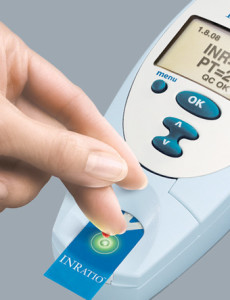
INRatio maker Alere’s gross profits slip in wake of nationwide recall of defective PT/INR monitor and test strips
Alere Inc. sees gross profits plunge over $100 million in 2014 In its March 2015 10k filing with the Securities and Exchange Commission (SEC), Alere, the maker of the INRatio PT/INR monitor system, revealed its gross profits in 2014 decreased $100.5 million compared to the previous year. The dive in annual gross profits represents an 8%…