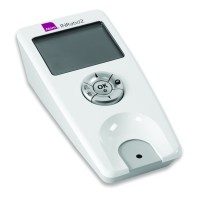
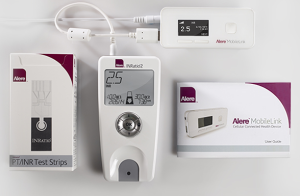
The Alere INRatio PT/INR monitor system is used by patients prescribed to the anticoagulant medication (blood thinner) warfarin. It is an electronic device powered by battery or power supply which tracks the PT/INR levels in a patient’s blood. Alere recently issued a recall of its INRatio and INRatio2 monitor systems because the medical devices provide inaccurately low INR results, putting users at significant risk of serious injuries and even death.
The Alere INRatio PT/INR monitor system is designed to operate by inserting a test strip into the device and then applying a drop of blood on the test strip. The Alere INRatio gives patients a digital reading of their PT/INR level. The Alere INRatio 2 is supposed to provide users the ability to connect the device to their home computer and email results to their healthcare providers.
What is warfarin?
Warfarin is a blood-thinning medication. It affects the body’s ability to form blood clots for therapeutic purposes. Warfarin is a long term treatment for the prevention of deadly blood clots. Common conditions which may produce life-threatening blood clots are deep vein thrombosis, pulmonary embolism, and atrial fibrillation.
Monitoring a patient’s PT/INR levels while taking warfarin is important to manage a balance between the prevention of blood clots and a severe bleeding events. If a patient’s PT/INR levels are higher than therapeutic levels severe bruising or bleeding may occur. If this occurs, an antidote must be administered to reverse the effects of warfarin and end the bleeding.
What is PT/INR?
 PT/INR stands for Prothrombin Time (PT) and International Normalized Ratio (INR). They are important because they measure the effectiveness of warfarin. PT assesses the way the human body reacts when a blood vessel is ruptured. INR is a reading given in seconds which shows how long plasma in the blood will take to form a clot.
PT/INR stands for Prothrombin Time (PT) and International Normalized Ratio (INR). They are important because they measure the effectiveness of warfarin. PT assesses the way the human body reacts when a blood vessel is ruptured. INR is a reading given in seconds which shows how long plasma in the blood will take to form a clot.
Laboratory plasma testing is the most accurate way of measuring PT/INR levels. The Alere INRatio PT/INR monitor system was developed as a way for patients to conveniently test their PT/INR levels at home, avoiding frequent trips to a doctor’s office.
What are therapeutic PT/INR levels?
For most patients taking warfarin, INR levels of 2.0 to 3.0 will produce adequate blood thinning to treat conditions associated with improper blood clotting. Patients at a high risk for deadly blood clots may have their INR levels set between 2.5 to 3.5. Based on these readings, a doctor will adjust the patient’s dosage of warfarin.
There are certain medical conditions and external factors which may affect the accuracy of PT/INR readings. Alere has issued a recall for its INRatio PT/INR monitor system because consumers with certain medical conditions were given inaccurate PT/INR readings. Consumers may have received PT/INR readings which they believed were otherwise within therapeutic levels but were actually inaccurate, putting them at risk for severe injury or death.
How inaccurate were PT/INR readings given by the Alere INRatio?

Consumers using the Alere INRatio PT/INR monitor system have reported INR readings significantly lower than those taken in laboratory plasma tests. Plasma blood tests taken in a clinical setting are the most accurate measurement of what a patient’s true INR levels are. INR readings taken in these clinical tests show consumers using the Alere INRatio monitoring system were receiving readings anywhere from 3.1 to 12.2 units lower than what their actual INR level.
For example, a consumer using the Alere INRatio may receive a safe INR reading of 2.5 when their INR may actually be as high as 14.7. The higher the INR level, the thinner a patient’s blood, increasing the likelihood of a severe and possibly deadly bleeding event.
These low INR readings given to consumers using the Alere INRatio monitoring device may put them at a high risk of injury since they believe their INR levels are safe when they are actually many times higher than a therapeutic level should be.
Alere received almost 19,000 reports of malfunction from users of its INRatio PT/INR monitor system. The FDA has designated the Alere INRatio PT/INR recall a Class I recall, meaning it is the most serious type of recall because consumers will likely be seriously injured or may die from using this medical device.
What are my options if I was severely hurt while using an Alere INRatio monitoring system?
If you were seriously injured while using an Alere INRatio PT/INR monitoring system, you may be eligible for substantial compensation. The Cochran Firm, D.C. can help to recover damages for lost wages, medical bills, and pain and suffering. Our office has a team of dedicated and experienced attorneys who are ready to help you get the compensation you deserve.
There are strict time deadlines when filing a claim so please contact us at your earliest convenience. Call us at 1-800-THE-FIRM or our local number at 202-682-5800. You can also fill out a contact form online. Our consultations are free, prompt, and confidential and there are no fees unless we recover for you.