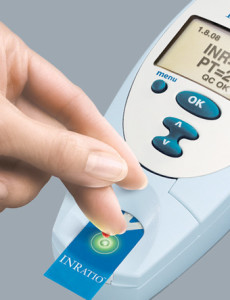
The Alere INRatio received FDA approval through a controversial fast track program
FDA’s 510(k) program allows medical device makers to skip clinical trials Under a little-known FDA process, medical device makers selling products similar to previously-approved devices are able to forego the burdensome and costly traditional approval process. The FDA’s Premarket Notification program allows medical device makers like Alere to sell their products to consumers more rapidly, but critics say…