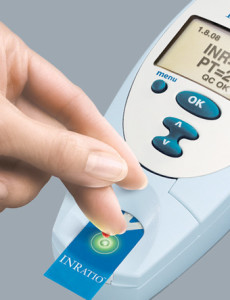
Alere’s INRatio INR monitor is under a Class I recall. What is an FDA Class I recall?
The Alere INRatio PT/INR monitor system and test strips are under Class I FDA recall The INRatio PT/INR monitor system is a medical device used to track blood clotting time (PT/INR) of patients prescribed warfarin. Warfarin is a blood thinning medication used in the prevention of clot-related conditions such as stroke and deep vein thrombosis. Patients…