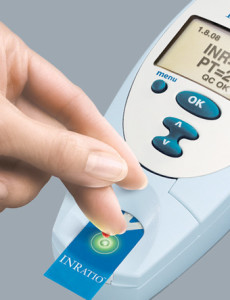
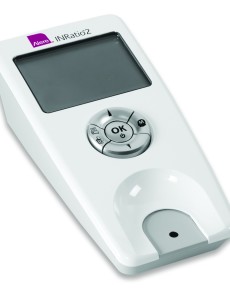
Alere INRatio monitor recalled for inaccurate PT/INR readings
Alere has issued two separate recalls associated with inaccurate INR readings. According to the FDA, the INRatio and INRatio2 Monitor Systems display inaccurate INR levels for some patients. The Alere INRatio may present INR readings at healthy levels when in fact the correct INR result is much higher when obtained by more-accurate traditional laboratory plasma…